so42 lewis structure|How To Draw The Lewis Structure of SO4 2 : Tagatay SO42- Lewis Structure, Hybridization, Bond Angle and Molecular Geometry. SO42- is a chemical name for the sulfate ion. It comprises one Sulphur atom, four Oxygen atoms, and a charge of -2. It . Get the latest news, photos, videos, and more on Philippines from Yahoo - Latest News & Headlines.
so42 lewis structure,A step-by-step explanation of how to draw the SO4 2- Lewis Dot Structure (Sulfate ion).For the SO4 2- structure use the periodic table to find the total numb. In this article, we have covered Lewis Structure, Molecular Geometry, Hybridization type, and nature of Polarity of SO42- in an extensive format. This will make your learning of sulfate ion all the way .
SO42- Lewis Structure, Hybridization, Bond Angle and Molecular Geometry. SO42- is a chemical name for the sulfate ion. It comprises one Sulphur atom, four Oxygen atoms, and a charge of -2. It .
Lewis Structure for SO4 2- (Sulfate Ion) Commonly Tested Lewis Structures. We draw Lewis Structures to predict: -the shape of a molecule. -the reactivity of a molecule and . This chemistry video explains how to draw the lewis structure of the sulfate ion SO4 2-.How To Draw Lewis Structures: https://www.youtube.com/wat.The SO4 2- (Sulfate Ion), comprised of one sulfur atom and four oxygen atoms, presents a captivating example of a chemical species with intriguing properties. At the heart of .The Lewis structure for SO 42- is requires you to place more than 8 valence electrons on Sulfur (S). You might think you've got the correct Lewis structure for SO 4 at first. . For today’s video, we are going to do SO42- Lewis Structure. It is a chemical formula for Sulfate ions. To determine its Lewis Structure, we first find out the total number of valence.
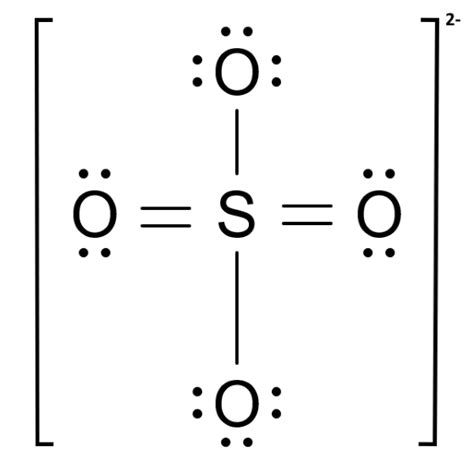
The SO42- Lewis structure depicts the molecular arrangement of sulfate, which consists of one sulfur atom and four oxygen atoms. The structure has two double bonds and two single bonds .
Steps of drawing SO4 2- lewis structure Step 1: Find the total valence electrons in SO4 2- ion. In order to find the total valence electrons in SO4 2- ion, first of all you should know the valence electrons present in sulfur atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any .
Sulfates (salts with the SO 4 2-) are frequently used in industry and biologically.. A commonly used sulfate is sodium lauryl ether sulfate found in shampoo, toothpaste, etc. For example, MgSO 4 is also known as Epsom Salts.. There are 32 valence electrons available for the Lewis structure for SO 4 2-.. Video: Drawing the Lewis Structure for SO 4 2-
Chủ đề so4 2- lewis structure Cấu trúc Lewis của SO4 2- giúp chúng ta hiểu rõ hơn về cách các nguyên tử trong ion này liên kết với nhau. Bài viết này sẽ cung cấp một hướng dẫn chi tiết, từng bước để vẽ cấu trúc Lewis của SO4 2-, từ việc tính toán số electron hóa trị đến xác định hình học phân tử và góc liên .The Lewis structure, proposed by Gilbert Newton Lewis, who introduced it for the first time in 1916, is a graphic representation of the sharing of electrons that occurs in chemical bonds between atoms of the same or different species. These bonds can .How To Draw The Lewis Structure of SO4 2 5 Steps to Draw the Lewis Structure of SO4 2- ion Step #1: Calculate the total number of valence electrons. Here, the given ion is SO4 2-.In order to draw the lewis structure of SO4 2-ion, first of all you have to find the total number of valence electrons present in the SO4 2-ion. (Valence electrons are the number of electrons present in the . The Lewis dot structure of a molecule is referred to as a simplified representation of all the valence electrons present in it. Therefore, the very first step while drawing the Lewis structure of [SO 4] 2- is to count the total valence electrons present in the concerned elemental atoms.. There are two different elements present in the sulfate . There are equivalent six resonance structures SO4 2- the Sulfate ion. We start with a valid Lewis structure and then follow these general rules.- Resonance .

There are two S = O bonds and two S-O bonds in the Sulfate ion Lewis structure. Lewis Dot Structure of NO 2-: Total valence electrons= Valence electron of Nitrogen + Valence electron of Oxygen; Valence electrons= 5 + 6 × 2 + 1 = 18; Due to one negative charge, one electron will be added.
There are two S = O bonds and two S-O bonds in the Sulfate ion Lewis structure. Lewis Dot Structure of NO 2-: Total valence electrons= Valence electron of Nitrogen + Valence electron of Oxygen; Valence electrons= 5 + 6 × 2 + 1 = 18; Due to one negative charge, one electron will be added.
In lewis structure of S 2 O 3 2-ion, there is -2 charge and oxygen atoms should hold them. Total valence electrons of sulfur and oxygen atoms are used to draw the structure. Thiosulfate ion | S 2 O 3 2-Thiosulfate ion is one of the oxyanion of sulfur. Two sulfur atoms exist at two different oxidation states as +4 and +6.
The initial step in drawing the SO 4 2-Lewis structure is to calculate the total number of valence electrons present in the molecule. Since sulfur and oxygen belong to group 16 of the periodic table, they each have six valence electrons. As SO 4 2-contains one sulfur atom and four oxygen atoms, the total number of valence electrons can be . The Lewis structure of SO 4 2-contains two single bonds and two double bonds, with sulfur in the center, and four oxygens on either side. The top oxygen atom and bottom oxygen atom has three lone .

Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.so42 lewis structure How To Draw The Lewis Structure of SO4 2Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha. A step-by-step explanation of how to draw the Sulfate Ion Lewis Dot Structure (SO42- ). We'll also look at the molecular geometry, bond angles, electron geo. Hint: For drawing the Lewis structure of any compound or molecule we have to know about the number of valence electrons present in that compound and the valency of each atom present in that compound. Complete step by step answer: For drawing the Lewis structure of Sulfate ion (${\text{SO}}_{\text{4}}^{{\text{2 - }}}$) we have to follow .
Hi Everyone! For today’s video, we are going to do SO42- Lewis Structure. It is a chemical formula for Sulfate ions. To determine its Lewis Structure, we fir.so42 lewis structureLewis structure of sulfate ion is drawn in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of SO42- ion. In lewis structure, there should be charges on atoms. This chemistry video explains how to draw the lewis structure of the sulfate ion SO4 2-.How To Draw Lewis Structures: https://www.youtube.com/wat.
Understanding the Lewis Structure of SO4 2-The first subtitle focuses on explaining the fundamental concepts behind the Lewis structure of the sulfate ion (SO4 2-) in the context of Mathematics education. It aims to provide a clear understanding of how to represent the molecule using Lewis dot diagrams.
so42 lewis structure|How To Draw The Lewis Structure of SO4 2
PH0 · SO42 Lewis Structure, Molecular Geometry,
PH1 · SO42
PH2 · SO4 2
PH3 · Lewis Structure for SO4 2
PH4 · How to Draw the Lewis Structure for the Sulfate Ion
PH5 · How To Draw The Lewis Structure of SO4 2